X-ray Absorption Edges (theory vs reality)
Theory isn't good enough - measure it yourself
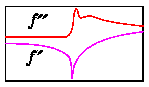
The Cromer/Liberman theoretical values for scattering factors are not accurate
for energies very near an absorption edge. Here the interaction of the
scattering atom with its chemical neighbors complicates the scattering
behaviour considerably. Shown below is a comparison of the theoretical values
of f' and f" with the values determined experimentally for the Cu
site in single crystal of a blue copper protein [Guss et al, 1989].
In both cases the f' values were derived from the corresponding f" spectra
via the Kramers-Kronig equation.
![[figure]](/scatter/cbpscan.gif)
Note in particular:
- The actual absorption edge is shifted relative to the idealized edge for an
isolated Cu atom. The largest part of this shift is due to the oxidation
state of the Cu atom in the protein.
- The local chemical environment introduces "ripples" (EXAFS) into the
scattering spectrum.
- The maximum achievable f" is actually larger than the edge jump from theory.
(The effect isn't very large in this particular example, but sometimes it is
substantial).
- The maximum achievable |f'|, however, is smaller than the theoretical value.
This is largely limited by the energy bandwidth of the x-ray source.
 X-ray Anomalous Scattering
X-ray Anomalous Scattering
 Breaking Friedel's Law
Breaking Friedel's Law
Ethan A Merritt ©1996-2001/ merritt@u.washington.edu /
Biomolecular Structure Center at UW
![[figure]](/scatter/cbpscan.gif)
![[figure]](/scatter/cbpscan.gif)
 X-ray Anomalous Scattering
X-ray Anomalous Scattering
 Breaking Friedel's Law
Breaking Friedel's Law